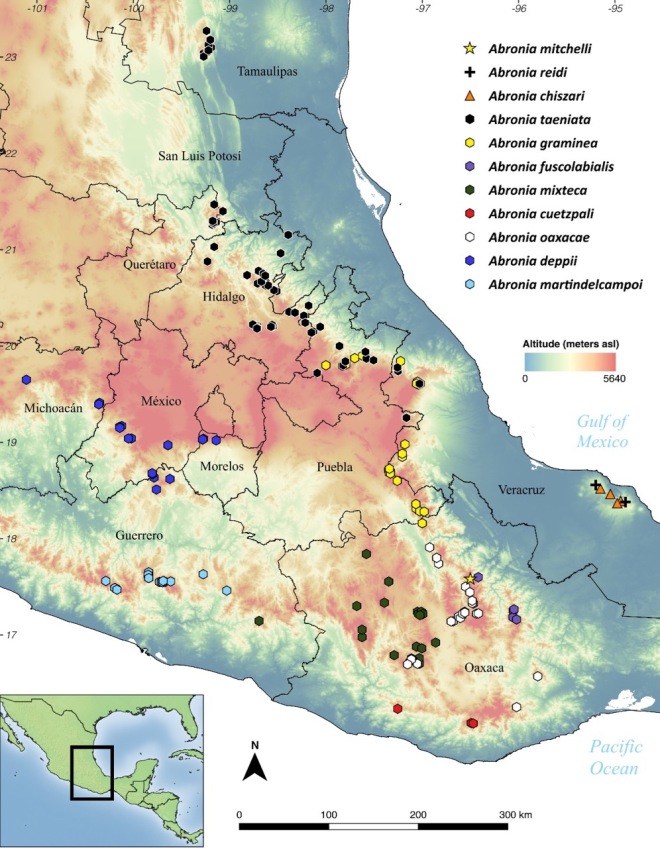
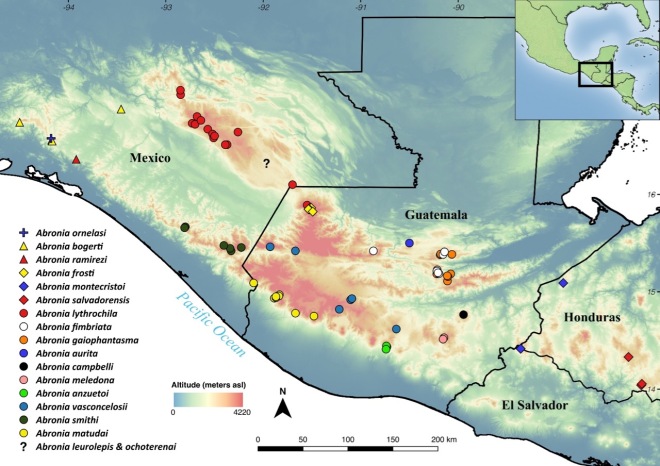
Determining how to prioritize resource-management interventions is hard. Conservation decision-making is often hampered by imperfect data, and rigorous monitoring to measure post-intervention outcomes is rare. These issues motivated a turtle study that my colleagues and I recently published. Although we worked in a non-Mexican study system, there are potential applications to Baja California and elsewhere in northern Mexico. Let me explain.
Our team examined the idea that the globally-invasive red-eared slider turtle, Trachemys scripta elegans, competes with the native, imperiled western pond turtle, Emys marmorata (the latter is shown in the banner photo for this post). The slider is listed as one of the world’s 100 most invasive species by the IUCN (Lowe 2000). Several experiments under captive conditions show that sliders can outcompete native turtles in the family Emydidae for a variety of resources (e.g., Cadi & Joly 2003, Polo-Cavia et al. 2011). But—and this is a big but—there was no data from wild, free-living populations. So, we decided to remove the majority of an introduced slider population (177 turtles!) from a well-studied waterway in northern California (see Spinks et al. 2003 and Lambert et al. 2013), and then monitor changes in basking habitat use and body condition of the co-occurring native western pond turtles.
 A non-native red-eared slider removed from a wetland in California, USA. The red “ear” marking on this particular turtle is unusually faint.
A non-native red-eared slider removed from a wetland in California, USA. The red “ear” marking on this particular turtle is unusually faint.
For the full story, please check out our paper (Lambert et al. 2019). You can read and download it for free here. But the takeaway message is: yes, red-eared sliders do compete with western pond turtles at this site. That said, the full story is a bit complicated. We found a strong signal of improved body condition in the western pond turtles after sliders were removed. This suggests that the two species compete for food. The basking behavior of the western pond turtles also changed post-removal. However, this change in behavior was inconsistent with what we expected if the two species were competing for basking sites. Furthermore, the western pond turtles basked a lot less post-removal, which was somewhat puzzling. Suffice it to say that my co-authors and I would love to see replication of this study in other systems. This is where Mexico comes in!
Outside of the USA, the genus Emys is native to much of northern Baja California. There is also an endemic Trachemys (the Baja California Slider, T. nebulosus) that occurs in the southern peninsula. Plus, range-restricted freshwater emydids exist elsewhere in northern Mexico, including Trachemys gaigeae, T. taylori, and T. yaquia. All of these lineages are of some conservation concern (Turtle Taxonomy Working Group 2017), and many populations occur in small, relatively isolated water bodies in arid environments (Grismer & McGuire 1993, Grismer 2002, Valdez-Villavicencio et al. 2016). So, they could be vulnerable to invasion by red-eared sliders. Or maybe this has already happened in some cases?
 Desert oasis near Mulegé, Baja California Sur, showing general habitat characteristics for some emydid turtle populations in northern Mexico.
Desert oasis near Mulegé, Baja California Sur, showing general habitat characteristics for some emydid turtle populations in northern Mexico.
A quick look on iNaturalist provides some clarity. The database reveals widespread, but clustered, observations of non-native sliders across northern Mexico, including Baja. Although imperfect, this information suggests that sliders now exist alongside range-restricted native turtles in the region. This raises two questions. First, are the non-native turtles having a detrimental effect on the native turtles? And if so, what are appropriate management actions to reduce this impact?
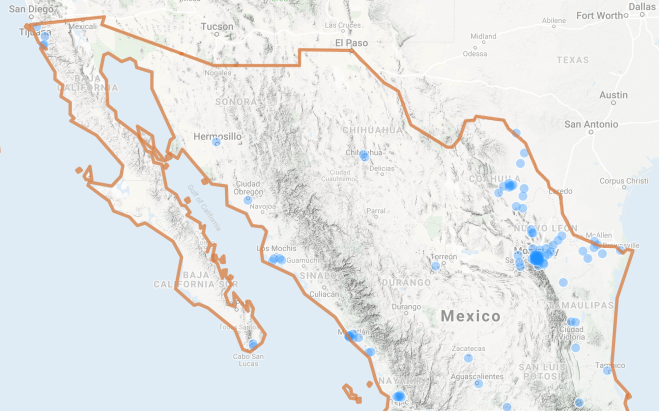
Answering these questions is a little tricky, but our recent paper provides some insight. One of our big messages is that controlling, much less eradicating, an established population of red-eared sliders is expensive and time consuming. Removing turtles is probably not a feasible approach for large, complex waterways (García-Díaz et al. 2017). Furthermore, without addressing the root cause of slider introductions (people releasing their unwanted pets), eradication or control campaigns will likely be ineffective in the long term. Nonetheless, invasion biology emphasizes the need to “catch things early.” So removing a handful of newly-introduced sliders from a small desert oasis occupied by native turtles could help prevent a minor problem from becoming a large, intractable problem.
Ultimately though, it is still unclear how harmful sliders actually are to native turtles in Mexico. Our California study is the first to look at whether sliders compete with native turtles in the wild. Are our results transferable to other sites, and to other native turtle species? Additional field experiments with pre- and post-removal monitoring are desperately needed! Fortunately, readers of this blog are perhaps best positioned to identify logistically-suitable places for such tests in Mexico. Such research is critical for figuring out the best way to prioritize our limited resources. Defensible turtle-management interventions depend on it.
My hope here is to help raise awareness of the need for rigorous evidence to support proposed management strategies. And perhaps spark ideas for new science that could improve Mexican turtle conservation practices. Please consider sharing your thoughts in the comments! We’d love to hear from you.
Literature Cited
Cadi A, Joly P. 2003. Competition for basking places between the endangered European pond turtle (Emys orbicularis galloitalica) and the introduced red-eared slider (Trachemys scripta elegans). Canadian Journal of Zoology 81:1392–1398
García-Díaz, P., D. S. L. Ramsey, A. P. Woolnough, M. Franch, G. A. Llorente, A. Montori, X. Buenetxea, A. R. Larrinaga, M. Lasceve, A. Álvarez, J. M. Traverso, A. Valdeón, A. Crespo, V. Rada, E. Ayllón, V. Sancho, J. I. Lacomba, J. V. Bataller, and M. Lizana. 2017. Challenges in confirming eradication success of invasive red-eared sliders. Biological Invasions 19:2739–2750.
Grismer, L. L. 2002. Amphibians and Reptiles of Baja California, Including its Pacific Islands and the Islands in the Sea of Cortés. University of California Press, Berkeley.
Grismer, L. L., and J. A. McGuire. 1993. The oases of central Baja California, México. Part I. A preliminary account of the relict mesophilic herpetofauna and the status of the oases. Bulletin of the Southern California Academy of Sciences 92:2–24.
Lambert, M. R., S. N. Nielsen, A. N. Wright, R. C. Thomson, and H. B. Shaffer. 2013. Habitat features determine the basking distribution of introduced red-eared sliders and native western pond turtles. Chelonian Conservation and Biology 12:192–199.
Lambert, M. R., J. M. McKenzie, R. M. Screen, A. G. Clause, B. B. Johnson, G. G. Mount, H. B. Shaffer, and G. B. Pauly. 2019. Experimental removal of introduced slider turtles offers new insight into competition with a native, threatened turtle. PeerJ 7:e7444.
Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the World’s worst invasive alien species. A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 12.
Polo-Cavia N, Lopez P, Martin J. 2011. Aggressive interactions during feeding between native and invasive freshwater turtles. Biological Invasions 13:1387–1396
Spinks, P. Q., G. B. Pauly, J. J. Crayon, and H. B. Shaffer. 2003. Survival of the western pond turtle (Emys marmorata) in an urban California environment. Biological Conservation 113:257–267.
Turtle Taxonomy Working Group [A. G. J. Rhodin, J. B. Iverson, R. Bour, U. Fritz, A. Georges, H. B. Shaffer, and P. P. van Dijk]. 2017. Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status (8th Ed.) inA. G. J. Rhodin, J. B. Iverson, P. P. van Dijk, R. A. Saumure, K. A. Buhlmann, P. C. H. Prtichard, and R. A. Mittermeier, editors. Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 7:1–292.
Valdez-Villavicencio, J. H., A. Peralta-García, and J. Á. Guillen-González. 2016. Nueva población de la tortuga de poza del suroeste Emys pallida en el Desierto Central de Baja California, México. Revista Mexicana de Biodiversidad 87:264–266.



 Sceloporus orcutti (left) and S. magister (right).
Sceloporus orcutti (left) and S. magister (right). Sceloporus formosus, El Sumidero, Veracruz
Sceloporus formosus, El Sumidero, Veracruz Sceloporus megalepidurus, El Sumidero, Veracruz
Sceloporus megalepidurus, El Sumidero, Veracruz Sceloporus grammicus, Altotonga, Veracruz
Sceloporus grammicus, Altotonga, Veracruz